Answer: The heat required will be 58.604 kJ.
Explanation:
To calculate the amount of heat required, we use the formula:
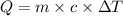
Q= heat gained or absorbed = ? J
m = mass of the substance = 100 g
c = heat capacity of water = 4.186 J/g ° C
Putting values in above equation, we get:
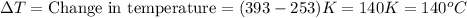
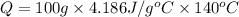
Q = 58604 Joules = 58.604 kJ (Conversion factor: 1 kJ = 1000J)
Thus, heat released by 100 grams of ice is 58.604kJ.