Answer: Option (D) is the correct answer.
Step-by-step explanation:
Parent atom is the atom which undergoes radioactive decay in a nuclear reaction. After the decay, this parent atom results in the formation of different products.
The given radioactive decay reaction will be as follows.
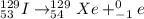
Here, beta particle is emitted along with the formation of
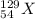 .
.
Thus, we can conclude that in this reaction the parent atom is
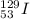 .
.