Answer: 5.72 g mass of carbon dioxide was produced
Step-by-step explanation:
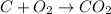
Moles of C =
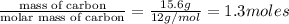
Mass of
 reacted = 59.1 g - 17.5 g = 41.6 g
reacted = 59.1 g - 17.5 g = 41.6 g
Moles of
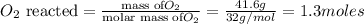
According to reaction 1 mole
 produces 1 mole of
produces 1 mole of
 then 1.3 mole of
then 1.3 mole of
 will produce
will produce
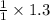 moles of
moles of

Mass of
 :
:
=Moles of
 × molar mass of
× molar mass of
 = 1.3 ×44 g/mol =
= 1.3 ×44 g/mol =
=57.2 grams
5.72 g mass of carbon dioxide was produced