Explanation :
An atom is a smallest particle of matter. An atom consists of protons, neutrons and electrons. These are three subatomic particles.
Protons are positively charged species while neutrons are negatively charged species. Electrons revolves around an atom.
Charge :
The charge of an electron is negative or
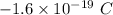
The charge of proton is +e or
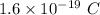
Neutron has no charge.
Mass :
Atomic mass of electron is
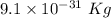
Atomic mass of proton is
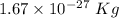
Atomic mass of neutron is
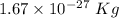
Both protons and neutrons are located inside the nucleus at the center.
Charge, atomic mass and location determines the properties of an atom. Atomic mass tells about the number of protons and neutrons inside an atom. Electric charge tells us the number of electrons gain or lost.