Here we have to judge the effect of tare of balance on the calculation of change of volume of a sample.
The change of calculated volume change depends on the proper taring of balance.
The volume of a solution or a compound depends on its weight and density. The relation between the volume (V) with weight (m) and density (d) is
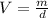 .
.
Thus if the proper taring of the balance is not done before taking the weight measurement of a particular sample the probability of getting exact weight of the compound is low and it will affect its volume. As the density of a particular sample remains same.
Now if the volume obtained is contain error the change of the volume for any experiment will also contain error and the obtained volume change will be differ from the exact change of volume.
Thus if the taring of the balance is not proper then the change of calculated volume will differ.