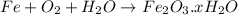Answer: Event 1 is an example of a physical change and Event 2 is an example of a chemical change.
Explanation: Physical change is one in which there is no change in chemical composition of the substance. There is only a change in phase change.
Chemical change is a change in which there is a change in chemical composition and there might or might not be a phase change.
On Boiling, the water molecules remain bonded in the same form and only covert from liquid to gaseous form, thus is a physical change.
On Rusting of iron nail, the iron changes to iron oxide by combining with oxygen, there is a rearrangement of atoms and thus is a chemical change.