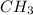atomic mass of carbon is C = 12 g/mol
atomic mass of hydrogen is H = 1 g/mol
now number of carbon atoms
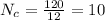
Similarly the number of hydrogen atoms are
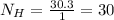
so Carbon atom and hydrogen atom must be in ratio of 10:30
so the ratio is 1:3
now the empirical formula is always in simplest form