Answer: The correct option is Option B.
Step-by-step explanation:
A balanced chemical equation is a chemical equation in which number of atoms on both the sides of the reaction remains same. All the balanced chemical equation follow Law of conservation of mass.
Law of conservation of mass states that the total mass in a chemical reaction remains same. The total mass on the reactant side will be same as the total mass on the product side.
From the given options:
Option A:
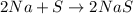
Here, total mass on the reactant side is not equal to the total mass on the product side. Hence, this equation is not a balanced chemical equation.
Option B:
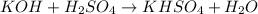
Total mass on the reactant side:
![[(39+16+1)+((2* 1)+32+(4* 16))]=154g/mol](https://img.qammunity.org/2020/formulas/chemistry/high-school/llqog3bmqghtypxtg43uyfngf0zzbw5izb.png)
Total mass on the product side:
![[(39+1+32+(4* 16))+((2* 1)+16)]=154g/mol](https://img.qammunity.org/2020/formulas/chemistry/high-school/kffshvuc2vv01wepguk4m613e7zc06v8ei.png)
As, total mass on the reactant side is equal to the total mass on the product side. Hence, this equation is a balanced chemical equation.
Option C:
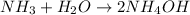
Here, total mass on the reactant side is not equal to the total mass on the product side. Hence, this equation is not a balanced chemical equation.
Option D:
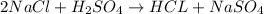
Here, total mass on the reactant side is not equal to the total mass on the product side. Hence, this equation is not a balanced chemical equation.
Hence, the correct answer is Option B.