According to Arrhenius concept a base is one which can donate hydroxide ion in its aqueous solution.
Here NaOH is a base as it can donate OH-.
As NaOH is a strong base it will have very high dissociation constant.
Due to high dissociation constant if will almost completely dissociate into sodium ions and hydroxide ions.
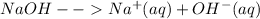
Thus in a solution of NaOH we will expect the following two ions
Na^{+} + OH^{-}[/tex]