Answer: The correct answer is

Step-by-step explanation:
The number of valence electrons that the elements belonging to Group IV contains are 4.
The electronic configuration for the elements belonging to group IV will be :
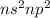
where, n is the number of period to which the element belongs.
Hence, the correct answer is
