Answer:The correct answer is option (C).
Step-by-step explanation:
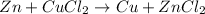
After the reaction between Zinc and copper(II) chloride, Zinc chloride and copper will be obtained as a product. This reaction occurs because zinc is more reactive than copper which displaces the copper from its aqueous solution.
The student will expect to find copper metal at bottom of the beaker with aqueous solution of zinc chloride.
Hence the correct answer is option (C).