Answer:
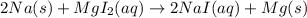
Step-by-step explanation: The chemical reaction is written by writing down the chemical formulas of the reactants on the left hand side and the chemical formulas of products on the right hand side separated by a right arrow.
This is a single displacement reaction in which a more reactive element displaces the less reactive element from its salt solution. Thus sodium is more reactive than Mg and thus displaces it from
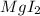 .
.
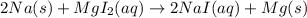
The number of atoms of each element must be same on both sides of the reaction so as to follow the law of conservation of mass. Thus the equation is balanced.