Answer: Because both the states are present at the given temperature.
Step-by-step explanation:
A physical equilibrium is defined as the system where physical states do not change when dynamic equilibrium is reached.
Ice and water are the solid and liquid states of water molecule.
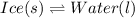
This is an example of physical equilibrium because both the states are present at this temperature and above 0°C, liquid state of water will be prominent and below 0°C, solid state of water which is ice will be prominent.