Answer:
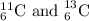
Explanation:
The general atomic symbol for an isotope is
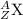
C has atomic number Z = 6, so it has six protons (p).
The mass number A =the number of protons plus neutrons = p + n
(1) Five neutrons
A = 6 + 5 = 11
The atomic symbol is
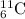 .
.
(2) Seven neutrons
A = 6 + 7 = 13
The atomic symbol is
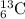 .
.