Answer:-
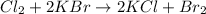
Explanations:- The first reaction is combustion reaction as the hydrocarbon is burned in presence of oxygen to give carbon dioxide and water.
Second reaction is the synthesis reaction as in general a synthesis reaction looks like:
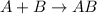
Third reaction is the decomposition reaction as it looks opposite of synthesis reaction.
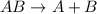
In general, a single displacement reaction looks like:
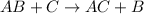
Fourth reaction is single replacement reaction as bromine is replaced by chlorine.
So, the correct choice is the last reaction,
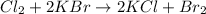 .
.