Answer:- B.
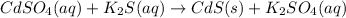
Explanations:- A precipitation reaction is a double displacement reaction in which a solid salt is formed.
If we look at all the given equations then it's only second reaction in which a solid salt (CdS) is formed. In rest of the reactions there is no solid salt formed.
It means A, C and D are not the right choices and so the correct choice is only B.
Here the phase are given for all the products. If the phases are not given then we use the solubility chart to see which reactions would form the precipitate.
Since, the precipitate is formed only in second equation, the correct choice is B.