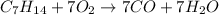Answer : The correct balanced equation will be,
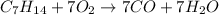
Explanation :
Balanced chemical equation : It is the number of atoms of individual elements present on reactant side must be equal to the number of atoms of individual elements present on product side.
As we know that the hydrocarbons can undergo complete or incomplete combustion that depends on the amount of oxygen available.
Complete combustion : It takes place when there is a good supply of oxygen. In this, hydrocarbon react with the oxygen to give carbon dioxide and water as a product.
Incomplete combustion : It takes place when there is a poor supply of oxygen. In this, hydrocarbon react with the oxygen to give carbon monoxide, carbon and water as a product.
As per question, the incomplete combustion of heptene react with oxygen to give carbon monoxide and water as a product.
Therefore, the balanced chemical reaction will be :