Answer: Chemical equation for the overall reaction obtained by adding these three given equations is:
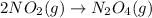
Step-by-step explanation:
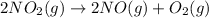 ...(1)
...(1)
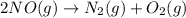 ...(2)
...(2)
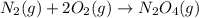 ...(3)
...(3)
If the same compounds is on same sides in the reactions then add them.
If the same compound is on different sides of the reaction then subtract.
Adding (1) and (2)
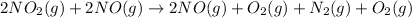
NO is present on both sides, which means it will get cancel out.
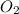 is on same side it will get added up.
is on same side it will get added up.
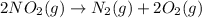 ..(4)
..(4)
Similarly, on adding (3) in (4) we get:
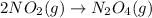
Above equation is obtained by adding given three equations.