Here we have to get the mass of the iron (Fe) produces in the reaction between ferric oxide (Fe₂O₃) and carbon monooxide (CO) to produce carbon dioxide and iron.
The amount of Fe produced in the reaction is B. 99.7g.
The balanced form of the reaction given is: Fe₂O₃ (s) + 3CO = 2Fe + 3CO₂.
Now as per the reaction 3 moles of CO produce 2 moles of Fe.
The molar mass of CO and Fe are 28.01g/mol and 55.845g/mol.
Thus (3×28.01) 84.03g of CO produces 111.69g of Fe.
Hence, 75.0g of CO produces
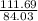 ×75.0 = 99.687g of Fe or 99.7g of Fe.
×75.0 = 99.687g of Fe or 99.7g of Fe.
A. If in the reaction 49.8g Fe is produced in the reaction then
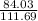 ×49.8 = 37.467g of CO should react. Thus the option is wrong.
×49.8 = 37.467g of CO should react. Thus the option is wrong.
C. To produce 224g of Fe
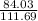 ×224 = 168.526g of CO will consumed. Thus it is wrong statement.
×224 = 168.526g of CO will consumed. Thus it is wrong statement.
D. To generate 299g of Fe
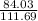 ×299 = 224.952g of CO will consumed. Thus it is wrong statement.
×299 = 224.952g of CO will consumed. Thus it is wrong statement.