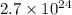Answer: The correct answer is
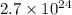
Step-by-step explanation:
We are given:
Moles of mercury = 4.5 moles
According to the mole concept:
1 mole of an element contains
 number of atoms.
number of atoms.
So, 4.5 moles of mercury will contain
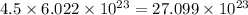 number of atoms.
number of atoms.
The rule that must be applied for the multiplication and division in significant figures is:
The least number of significant figures in any number of the problem determines the number of significant figures in the answer.
Here, the least precise significant number in problem are 2. Thus, the answer must also contain 2 least significant numbers.
Therefore, the correct answer becomes