Answer:
-
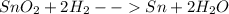
-
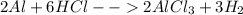
Step-by-step explanation:
Hello,
Single displacement reactions happen when one compound reacts with a single element or molecule to yield one compound and a single element or molecule as well by exchanging the cation or the anion depending on the case, in such a way, the reactions that accomplish the definitions are:
-
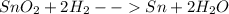
-
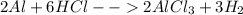
Best regards.