We have to calculate the percentage composition of the compound.
The answer is: amount (in mass percentage) of oxygen is 88.8 % and amount (in mass percentage) of hydrogen is 11.2 %.
We know atomic mass of oxygen is 16 and atomic mass of hydrogen is 1.
The amount of Oxygen produced= 19.98 g and amount of Hydrogen produced= 2.52 g. So, total amount of the compound is: (19.98 + 2.52) g= 22.50 g.
So, mass percentage of oxygen is (Mass of oxygen produced/ total mass of compound) X 100 =
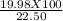 = 88.8 %
= 88.8 %
Masspercentage of hydrogen is: (Mass of hydrogen produced/ total mass of compound) X 100 =
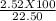 = 11.2 %.
= 11.2 %.