Here we have to get the effect of addition of 0.25 moles of gas C on the mole fraction of gas A in a mixture of gas having constant pressure.
On addition of 0.25 moles of C gas, the mole fraction of gas A will be
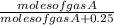 .
.
The partial pressure of gas A can be written as
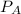 =
=
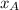 ×P (where
×P (where
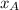 is the mole fraction of gas A present in the mixture and P is the total pressure of the mixture.
is the mole fraction of gas A present in the mixture and P is the total pressure of the mixture.
The mole fraction of gas A in a mixture of gas A and C is =
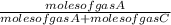 and
and
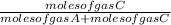 respectively.
respectively.
Thus on addition of 0.25 moles of C gas, the mole fraction of gas A will be
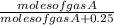 .
.
Which is different from the initial state.