Step-by-step explanation:
When heat is absorbed by reactants in a chemical reaction then the reaction is known as endothermic reaction.
Whereas when heat is released by reactants in a chemical reaction then the reaction is known as exothermic reaction.
1. When
 is positive then it means heat is absorbed by reactants and reaction is endothermic.
is positive then it means heat is absorbed by reactants and reaction is endothermic.
2. When
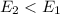 , this means energy of products is less than the energy of reactants. Therefore, it is exothermic in nature.
, this means energy of products is less than the energy of reactants. Therefore, it is exothermic in nature.
3. When energy is absorbed then reaction is endothermic.
4. When
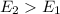 , this means energy of products is greater than the energy of reactants. Therefore, it is endothermic in nature.
, this means energy of products is greater than the energy of reactants. Therefore, it is endothermic in nature.
5. When [tex]\Delta H is negative then it means heat is released by reactants and reaction is exothermic.