Step-by-step explanation:
Since Charles found that the volume is directly proportional to temperature when the mass and pressure remain constant this means that with increase in temperature, there will be increase in volume.
Mathematically,
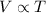 at constant Pressure
at constant Pressure
This equation represents the relationship in Charles' law.