Answer:
The pressure in Kilopascal is 24.28 kPa
Step-by-step explanation:
The rule of three or is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them. That is, what is intended with it is to find the fourth term of a proportion knowing the other three. Remember that proportionality is a constant relationship or ratio between different magnitudes.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied. To solve a direct rule of three, the following formula must be followed:
a ⇒ b
c ⇒ x
Then
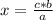
Being 1 atmosphere = 101.325 kilopascal, the rule of three applies as follows: if 1 atm equals 101.325 kPa, 0.2396 atm how many kPa do they equal?
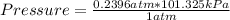
Pressure=24.28 kPa
The pressure in Kilopascal is 24.28 kPa