Answer:
4.61 atm
Step-by-step explanation:
This question seeks to test our knowledge on conversion of millimeter of mercury (mmHg) to atmospheric pressure (atm).
The numeric relationship between mmHg and atm is given below
1 atm ⇒ 760 mmHg, hence
X atm ⇒ 3,506 mmHg (where X is the unknown)
If you cross multiply the above, you will have
X =
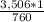
X = 4.61 atm
Thus, 4.61 atm is the atmospheric pressure of the gas exerted on the cylinder