Answer: 4.613 atm
Step-by-step explanation:
Pressure of the gas is defined as the force exerted by the particles on the walls of the container. It is expressed in various terms like 'mmHg', 'atm', 'kiloPascals' etc..
All these units of pressure are inter convertible.
We are given:
Pressure of the gas = 3506 mmHg
Converting this unit of pressure into 'atm' by using conversion factor:
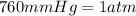
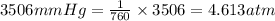
Thus there will be 4.613 atm in 3506 mmHg.