Answer:

Step-by-step explanation:
Displacement reaction:
- A reaction in which an element displaces or replaces another element of a compound is called a displacement reaction.
Types:
There are 2 types:
1. Single displacement reaction:
- If one element displaces 1 other element of a compound, it is called single displacement reaction.
- Example:
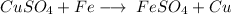
- Here, 1 element (Fe) displaces 1 other element (Cu) of a compound.
2. Double displacement reaction:
- If two elements in two compounds displace one another, it is called double displacement reaction.
- Example:
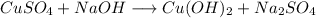
- Here, Copper and sodium both displace each other.
![\rule[225]{225}{2}](https://img.qammunity.org/2023/formulas/english/college/eq413d752mwtrwwenrzwldxt4w1olmf1b3.png)