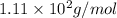Answer: The molar mass of the given compound is
Step-by-step explanation:
In molecular formula of
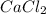 there are two chlorine atoms and one calcium atom.
there are two chlorine atoms and one calcium atom.
The atomic mass of the calcium = 40.07 g/mol
The atomic mass of chlorine = 35.5 g/mol
Molar mass of the guven compound is :
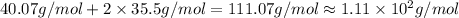
The molar mass of the given compound is