Answer: True
Step-by-step explanation:
A mole is defined as the amount of substance that contains Avogardro number of the substance. Avogadro's number is given by
 .
.
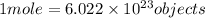
According to avogadro's law, 1 mole of every substance occupies 22.4 Liters at STP and contains avogadro's number
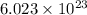 of particles.
of particles.
Thus the statement saying Avogadro's number is the number of particles in one mole of a pure substance is true.