Answer:- Mole ratio of D to A is 4:3.
Explanations:- Mole ratio for a chemical reaction is the ratio of the coefficients.
The given generic chemical reaction is:
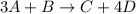
The numbers written in front of each chemical species in the chemical reaction are their moles. For the given generic chemical reaction the coefficient of A is 3 and that of B is 1. So, the mole ratio of A to B is 3:1.
Similarly if we want to write the mole ratio of C to D then it is 1:4.
We are asked to write the mole ratio of D to A. So, like the other ratios, the mole ratio of D to A is 4:3 as the coefficient of D is 4 and A is 3.