Answer: The bond between boron and hydrogen in boron trihydride is covalent bond.
Step-by-step explanation:
The type of bonding between the atoms forming a compound is determined by using the electronegativity difference between the atoms. According to the pauling's electronegativity rule:
- If
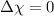 , then the bond is non-polar.
, then the bond is non-polar. - If
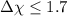 , then the bond will be covalent.
, then the bond will be covalent. - If
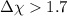 , then the bond will be ionic.
, then the bond will be ionic.
We are given:
Electronegativity for boron = 2.0
Electronegativity for hydrogen = 2.1
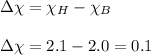
As,
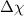 is less than 1.7 and not equal to 0. Hence, the bond between boron and hydrogen is covalent bond.
is less than 1.7 and not equal to 0. Hence, the bond between boron and hydrogen is covalent bond.