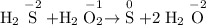
Step-by-step explanation
Find the oxidation state for each element.
The oxidation state of H is +1 in most compounds.
- The S atom in H₂S is bonded to two H atoms. The oxidation state of S in H₂S will be -(2 × (+1)) = -2.
- Two O atom in H₂O₂ are bonded to two H atoms. On average, the oxidation state for each O atom in H₂O₂ will be - 1/2 × (2 × (+1)) = -1.
- S has an oxidation state of 0 when it is not bonded to any other element;
- The O atom in H₂O is bonded to two H atoms. The oxidation state of O in H₂O will be -(2 × (+1)) = -2.
Changes in oxidation states:
- S: from -2 to 0.
- O: from -1 to -2.
Each S atom will reduce two O atoms. There are two O atoms in each H₂O₂ molecule. There's only one S atom in each H₂S molecule. As a result, pairing one H₂O₂ with one H₂S will balance the change in oxidation state.
Each H₂O₂ contains two O atoms. The two O atoms will produce two H₂O molecules. Each H₂S molecule will lead to one S molecule. Thus the coefficient for H₂O will be two and the coefficient for the rest three species will be one.