Answer: C)
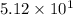 L
L

Explanation:
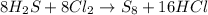
According to Avogadro's law, 1 mole of every gas occupies 22.4 Liters at STP.
Thus from the balanced chemical equation, 8 moles of hydrogen sulfide reacts with 8 moles of chlorine gas.
i.e 8 moles of hydrogen sulfide reacts with
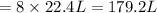 of chlorine gas.
of chlorine gas.
2.28 moles of hydrogen sulfide reacts with
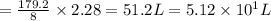 of chlorine gas.
of chlorine gas.