Answer:- Na = 27.37%, H = 1.20%, C = 14.30% and O = 57.14%
Solution:- For the percentage composition of a compound, the atomic mass of each atoms times its subscript is divided by the molar mass of the compound and multiplied by 100.
The given compound is
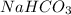 .
.
mass of Na = 22.99 g
mass of H = 1.008 g
mass of C = 12.01 g
mass of O = 3(16) = 48 g
Molar mass of compound = 22.99 g + 1.008 g + 12.01 g + 48 g = 84.008 g
percentage of Na =
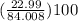
= 27.37%
percentage of H =
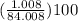
= 1.20%
percentage of C =
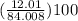
= 14.30%
percentage of O =
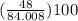
= 57.14%