Answer: 0.0211 moles of air are present inside the bottle
Step-by-step explanation:
Volume of the bottle = 0.500 L
Standard Pressure exerted by the air = 1 atm
Temperature of bottle in which air is present = 15 °C= 288 K(0 °C = 273 K)
Assuming air inside the bottle is behaving ideally:
PV=nRT
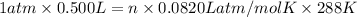
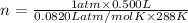
n = 0.0211 moles
0.0211 moles of air are present inside the bottle