Here we have to get the amount of water evaporated which generate 96.3 kJ of heat at boiling condition.
The amount of water evaporated is 23.043 g.
We know H = m×s×t, where H = generated heat, m = mass of the material, s = specific heat of the material, t = temperature.
We know the specific heat of water is 4.179 J/g°C.
At boiling condition of water the temperature is 100°C.
The heat generated 96.3 kJ or 9630 J.
On plugging the values we get,
9630 J = m×4.179 J/g°C × 100°C
Thus, 9630 J = m × 417.9 J/g
m =
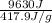
m = 23.043 g.