Answer: One substance which is slippery and conducts electricity in water is probably a base. The other substance which continuously made bubbles of hydrogen gas when Jake dropped magnesium into an aqueous solution of the substance is probably a acid.
Explanation: Bases are those substances which donates
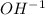 ions when dissolved in water. Thus bases produce ions which help in the conduction of electric current.
ions when dissolved in water. Thus bases produce ions which help in the conduction of electric current.
Bases are slippery in nature and are used in manufacturing of soaps.
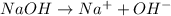
Acids are those substances which donates
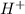 ions when dissolved in water. Thus acids also produce ions which help in the conduction of electric current. Acids are not slippery in nature.
ions when dissolved in water. Thus acids also produce ions which help in the conduction of electric current. Acids are not slippery in nature.
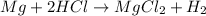
Thus magnesium when dropped into an aqueous solution of the acid releases hydrogen gas.