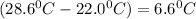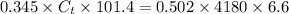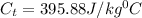Answer:
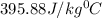
Explanation: Heat lost will be equal to the heat gained.
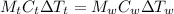
where
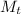 = mass of tungsten = 0.345 kg
= mass of tungsten = 0.345 kg
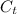 = specific heat of tungsten
= specific heat of tungsten
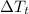 = Change in temperature of tungsten =
= Change in temperature of tungsten =
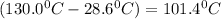
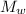 = mass of water = 0.502 kg
= mass of water = 0.502 kg
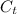 = specific heat of water =
= specific heat of water =
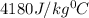
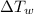 = Change in temperature of water =
= Change in temperature of water =