Answer: Option (d) is the correct answer.
Step-by-step explanation:
Kinetic energy is defined as the energy obtained by an object due to its motion.
Kinetic energy is directly related to temperature as follows.
Kinetic energy =
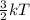
where, k = boltzmann constant
T = temperature
So, whenever there will be increase in temperature then there will be increase in kinetic or average kinetic energy of molecules of a substance.
This is because increase in temperature causes movement in the molecules of a substance. Due to this there will be increase in number of collisions.
Thus, we can conclude that increasing temperature of the object can be used to increase the average kinetic energy of the molecules of an object.