Answer:- C = 82.66% and H = 17.34%
Solution:- To calculate the percentage composition we divide the mass of each atom in the molecule by the molar mass of the molecule and multiply by 100.
The given molecule is
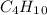 . It has four carbons and 10 hydrogens. So, to get the mass of carbon we multiply the atomic mass of C by 4. Similarly to get the mass of hydrogen we multiply the atomic mass of H by 10.
. It has four carbons and 10 hydrogens. So, to get the mass of carbon we multiply the atomic mass of C by 4. Similarly to get the mass of hydrogen we multiply the atomic mass of H by 10.
mass of C = 4(12.01) = 48.04 g
mass of H = 10(1.008) = 10.08 g
Molar mass of molecule = 48.04 g + 10.08 g = 58.12 g
mass percentage of C =
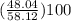
= 82.66%
mass percentage of H =
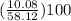
= 17.34%
So,
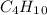 has 82.66% carbon and 17.34% hydrogen by mass.
has 82.66% carbon and 17.34% hydrogen by mass.