Step-by-step explanation:
Molar mass is the total mass number of all the atoms present in a compound or formula in grams per mole.
Molar mass of
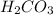 is as follows.
is as follows.
Molar mass of
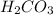 = 2 × Mass of hydrogen atom + mass of carbon atom + 3 × mass of oxygen atom
= 2 × Mass of hydrogen atom + mass of carbon atom + 3 × mass of oxygen atom
= 2 × 1.007 g/mol + 12.010 g/mol + 3 × 16 g/mol
= (2.014 + 12.010 + 48) g/mol
= 62.024 g/mol
Thus, we can conclude that molar mass of
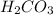 is 62.024 g/mol.
is 62.024 g/mol.