Adiabatic process is that process in which heat of a closed system will remain conserved or we can say that system can not exchange its thermal energy from its surrounding
So here from 1st law of thermodynamics we can say
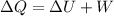
since we know that
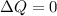
so we will have
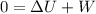
so we have
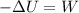
so here correct answers are
A. -ΔU = W
B. Heat is constant.