Answer:
Balanced Equation for reaction between Magnesium and Oxygen:
2Mg + O₂ --> 2MgO
Molar Mass of MgO is the atomic masses listed on the periodic table for the two elements Magnesium and Oxygen. Magnesium's molar mass is 24g/mol, and Oxygen's molar mass is 16g/mol. So MgO's molar mass would be 24 + 16 = 40g/mol.
The equation to find moles is:
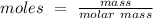
So if we rearrange this equation to find for mass:
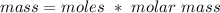
So you have to multiply 0.300 moles by 40, which gives you 12g
Meaning the mass of Magnesium Oxide is 12g.