Answer: The correct answer is strontium, calcium, magnesium.
Step-by-step explanation:
Reactivity is defined as the tendency of an atom to loose or gain electrons.
The reactivity of metal decreases as we move from left to right in a period and increases as we move from top to bottom in a group.
The given elements are metals and they belong to group 2 of the periodic table.
Calcium belongs to Period 4 of the periodic table, magnesium belongs to Period 3 of the periodic table and strontium belongs to Period 5 of the periodic table.
Strontium will be more reactive than calcium and it will be more reactive than magnesium.
Order of reactivity of metals follows:
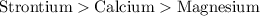
Hence, the correct answer is strontium, calcium, magnesium.