Answer: The 234.74 grams of sample should be ordered.
Step-by-step explanation:
Let the gram of 114 Ag to ordered be
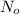
The amount required for the beginning of experiment = 0.0575 g
Time requires to ship the sample = 4.2hour = 252 min(1 hr = 60 min)
Half life of the sample =
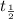 = 21 min
= 21 min
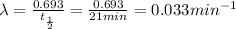
![\log[N]=\log[N_o]-(\lambda t)/(2.303)](https://img.qammunity.org/2020/formulas/chemistry/high-school/o2xfx99aycbbozxd1odqqt5swf89xb8jm4.png)
![\log[0.0575 g]=\log[N_o]-(0.033 min^(-1)* 252 min)/(2.303)](https://img.qammunity.org/2020/formulas/chemistry/high-school/705s4zr38abjm45iet956p7ol5dy8d2e6u.png)
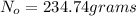
The 234.74 grams of sample should be ordered.