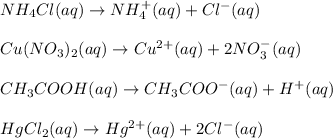Answer :
(1) The volume must dilute to make 500 mL of a 0.50M NaCl solution is, 0.125 L
(2) The amount of
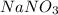 precipitate will be, 52 grams
precipitate will be, 52 grams
(3) The ionized equations are,
Solution for Part 1 :
Formula used :

where,
 = concentration of NaCl stock solution = 2 M = 2 mole/L
= concentration of NaCl stock solution = 2 M = 2 mole/L
 = concentration of NaCl solution = 0.50 M = 0.50 mole/L
= concentration of NaCl solution = 0.50 M = 0.50 mole/L
 = volume of NaCl stock solution
= volume of NaCl stock solution
 = volume of NaCl solution = 500 ml
= volume of NaCl solution = 500 ml
Now put all the given values in the above formula, we get the volume of NaCl stock solution.
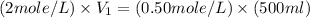
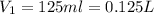 (1 L = 1000 ml)
(1 L = 1000 ml)
Therefore, the volume must dilute to make 500 mL of a 0.50M NaCl solution is, 0.125 L
Solution for Part 2 :
First we have to calculate the mass of
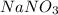 at
at
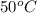 .
.
In 100 grams of water, the amount of sodium nitrate = 114 g
In 200 grams of water, the amount of sodium nitrate =
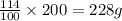
Now we have to calculate the mass of
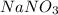 at
at
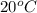 .
.
In 100 grams of water, the amount of sodium nitrate = 88 g
In 200 grams of water, the amount of sodium nitrate =
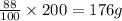
Now we have to calculate the amount of sodium nitrate precipitated.
The amount of sodium nitrate precipitated = 228 - 176 = 52 g
Therefore, the amount of
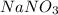 precipitate will be, 52 grams
precipitate will be, 52 grams
Solution for Part 3 :
When the substance dissolved in water then they disassociate into respective ions.