Answer :
Explanation for part 1 :
1 M solution means that the one mole of solute present in one liter of solution and also known as molarity of the solution. The unit of molarity is mole/liter.
1 m solution means that the one mole of solute present in one kilogram of solvent and also known as molality of the solution. The unit of molality is mole/kilogram.
Explanation for part 2 :
Given : Moles of KCl (solute) = 1 mole
Volume of solution = 750 ml
Formula used for molarity :
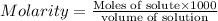
Now put all the given values in this formula, we get the molarity of solution.
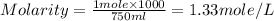
Therefore, the molarity of the solution is, 1.33 mole/L
Explanation for part 3 :
Part (a) : Given,
Mass of ammonium chloride (solute) = 0.54 g
Volume of solution = 250 ml
Molar mass of ammonium chloride = 53.491 g/mole
First we have to calculate the moles of ammonium chloride.
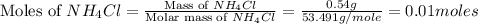
Now we have to calculate the concentration of solution.
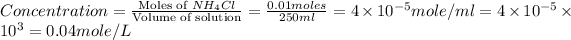
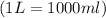
Therefore, the molarity of the solution is, 0.04 mole/L
Part (b) : Given,
Mass of sodium phosphate (solute) = 492 g
Volume of solution = 500 ml
Molar mass of ammonium chloride = 163.94 g/mole
First we have to calculate the moles of sodium phosphate.
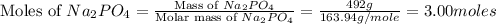
Now we have to calculate the concentration of solution.
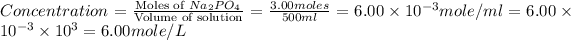
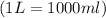
Therefore, the molarity of the solution is, 6.00 mole/L