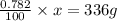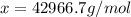Answer: 42966.7 g/mol
Step-by-step explanation: As 1 molecule of protein contains 3 atoms of Cd.
1 mole of protein contains=
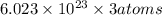 =3 moles of Cd.
=3 moles of Cd.
1 mole of Cd weighs= 112 g
3 moles of Cd weigh=
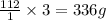
Given: 0.782% of Cd is present by mass. Thus if x is the molar mass of protein,